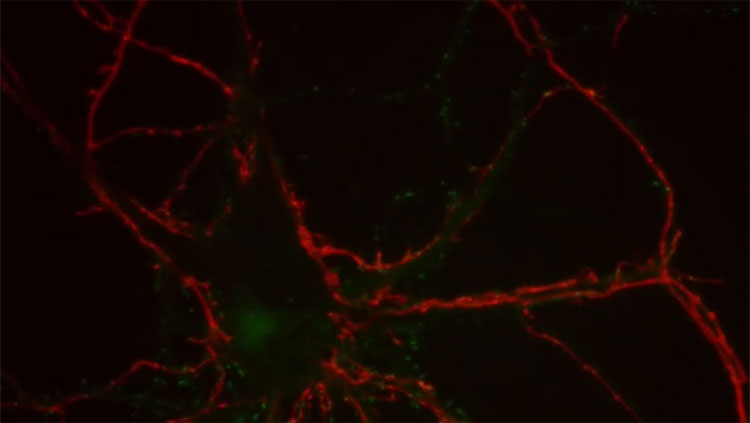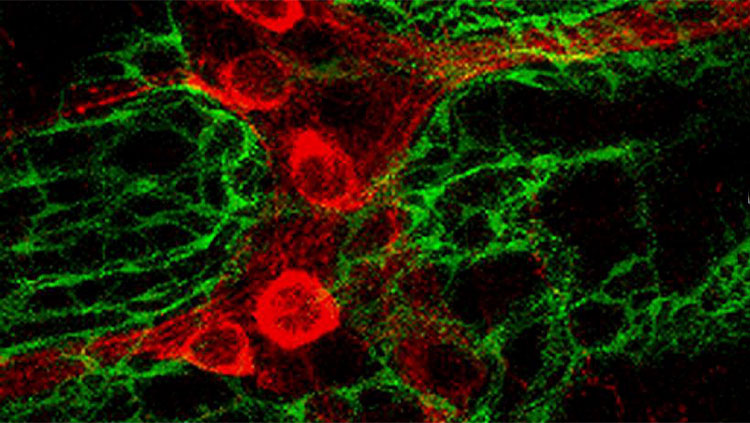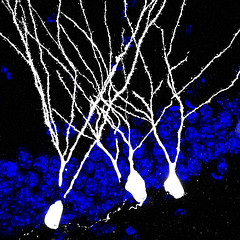
You know what’s nuts? That we still don’t know the basics. Forget patch clamping thousands of neurons in vivo during awake behavior. Simultaneously. In humans. In outer space. I just want to know exactly which other neurons my neuron is talking to. Not even. Which neurons is my neuron touching, intimately, in a putative synaptic contact sort of way? WHICH NEURONS MY NEURON DONE BEEN TOUCHING, YA DIG??
The problem doesn’t sound like it should be so difficult to answer. To use a completely accurate analogy, our brain is like a mass of spaghetti that has been left to congeal. Why not just follow the noodles (neurons) from one end to another? Well, because there are billions of them! And because these are darn small noodles! If you can label an individual neuron in vivo you might get away with it - you can cut the brain into slices and trace that individual neuron across the slices and reconstruct it (although maybe with this strategy neuronal reconstruction will be a thing of the past). But labeling individual neurons in vivo is hard and inaccurate. Plus, this will only tell you where the neuron projects, but not if it is actually making synaptic contacts.
Anterograde and retrograde tracers are another strategy. Here, a molecule is taken up by neurons and transported down axons to target regions, or taken up by axons and transported upstream. However, they label populations of neurons indiscriminately. Since many brain regions are composed of different cell types it becomes impossible to know exactly which types of neurons have taken up the tracer and transported it. Identifying these connectivity patterns is important for understanding how various neural processes, mediated by different brain structures, come together to give us behavior.
This is where genetics enters the picture. Each type of cell is controlled by a unique genetic program. By hijacking these programs, fluorescent proteins can now be expressed in specific cell types, allowing their axons to be traced and connections to be identified. In the hippocampus, or any region where neurons are born throughout adulthood, we are faced with a unique variant of this problem because we not only have two types of closely intermingled cells but they are genetically very related - they’re the same class of neuron, it’s just that they’re different ages. So it’s pretty impressive that some folks have discovered a way to specifically label adult-born neurons, but also identify their presynaptic partners. And the qualitative observations alone are pretty surprising and, if they hold up, should be seriously changing how we think about the function of adult neurogenesis.
In the recent1 study by Vivar et al., the inputs onto adult-born neurons were tracked throughout their development, from 21 days to 90 days after mitosis. Their strategy was to infect adult-born neurons with rabies virus, which travels retrogradely through the nervous system by crossing synapses and infecting upstream neurons. Since the brain is vastly interconnected, the challenge is to limit the spread of the infection. With a normal rabies virus, it would be impossible to distinguish neurons that were immediately upstream of the original infection from neurons that were infected by a polysynaptic pathway. The trick is then to limit the virus such that it can cross the first synapse but not any additional synapses. You can read about the details of their method in their recent review, from which I’ve borrowed the following figure.
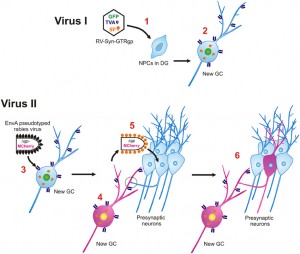
Briefly, they first injected a retrovirus into the dentate gyrus, which selectively infects dividing cells (i.e. precursor cells and their offspring daughter cells). This caused new neurons to express green fluorescent protein, the “TVA” receptor, and rabies glycoprotein. Later (21-90d) they injected a modified rabies virus into the dentate gyrus that lacks the rabies glycoprotein gene (necessary to cross synapses), only binds to cells that express the TVA receptor (i.e. the previously-infected adult-born neurons), and causes expression of the red fluorescent protein, mcherry. The result is that some adult-born cells are infected by both viruses and can be identified because they fluoresce both green (from the retrovirus) and red (from the rabies virus). These cells are dubbed ‘starter cells’. Importantly, in these doubly-infected starter cells, the rabies virus can take advantage of the glycoprotein offered by the retrovirus and it can therefore cross one afferent synapse and label upstream neurons, dubbed ‘traced cells’. Since the rabies virus itself does not possess the glycoprotein gene, retrograde synaptic transmission stops there. But the rabies virus will continue to cause expression of red fluorescent protein in these upstream neurons. Vivar could then scan the brain for the traced cells and identify the immediate presynaptic partners of adult-born neurons.
So who were these guys?
Mature dentate gyrus neurons! Whaaaaa? Dentate gyrus neurons synapse onto themselves? Hippocampal region CA3 is well known for its recurrent circuitry - it is thought that feedback excitation enables retrieval of a complete memory when only a subset of CA3 pyramidal neurons are initially active. But this type of circuitry has never been described within the dentate gyrus. It’s not likely that this is the main function of the dentate gyrus though, because this intra-dentate circuit is short-lived: 21 and 30 day-old, but not older, neurons received inputs from mature neurons. I am very curious as to the significance of this finding. It suggests that adult-born neurons could initially be recruited into memory traces that also involve older neurons, but that they might then become independent with time. Would this contribute to the transformation of memory, enabling a memory to evolve through different traces? Or might it just provide a mechanism by which, when there is a lot of hippocampal activity, mature neurons can ensure the survival of immature neurons for future encoding events? Or ensure the death of adult-born neurons if pre-existing neurons have the encoding situation under control? We could speculate indefinitely because...we really have no clue2.
Cholinergic septal neurons. Not weird since we know that the cholinergic system is heavily involved in memory. But this synapse is not the first thing that pops into my mind when I think about the circuit function of adult neurogenesis. And maybe it still shouldn’t be. But it could now be as high as the 3rd-6th thing that pops into my head when I think about the circuit function of adult neurogenesis. A little respect for acetylcholine, please.
NOT medial entorhinal cortical neurons. This is nuts. Every single3 electrophysiological study of adult-born neurons has said something like this in the methods: “Stimulating electrodes were placed in the middle third of the molecular layer to activate the medial perforant path...” And now we find out that maybe nobody was actually stimulating this pathway at all? Vivar found that adult-born cells only receive inputs from the lateral entorhinal cortex4, which is often thought to convey object-related information to the hippocampus, as opposed to information about the greater spatial context that is carried by the medial entorhinal cortex. This is the type of finding that suggests that the adult-born population might truly serve a unique function, and initially I was a little skeptical of this finding. But the authors did a number of experiments to support this finding, and found that lesions of the medial entorhinal cortex had minimal effects on synaptic transmission onto adult-born cells. In contrast, lesions of the lateral entorhinal cortex had much stronger physiological effects and also led to behavioral deficits in pattern separation that are similar to those observed when adult neurogenesis is inhibited.
CA3 pyramidal neurons. This is pretty interesting since CA3 is typically thought to be downstream from the dentate gyrus. Previous studies have suggested that CA3 backprojects to the dentate gyrus. So while this finding is not entirely new, it uses a new technique to confirm this generally underappreciated component of hippocampal circuitry. This pathway may even now join the club called the Top 10 to 20 Synaptic Pathways That Jump to Mind When People Think About Hippocampal Circuits.
Hilar neurons, both inhibitory and excitatory. More evidence that local circuitry feeds back onto adult-born cells and probably plays a big role in their development and function. Maybe it’s time to get rid of all those trisynaptic pathway schematics.
Astrocytes. Astrocytes form synapses? These inputs develop with age, paralleling the formation of synapses from the cortex. Indeed, preliminary structural evidence indicates that astrocytes are closely involved in synapse formation onto new neurons. Do they actually form synapse-like inputs? Or do they participate in structural remodeling and take up the rabies virus by virtue of being so close to the synapse?
The numbers.
Identifying the types of cells that synapse onto adult-born neurons allows for a lot of theoretical speculation, and is a major advance. But we will obviously need to go deeper and identify the physiological properties of these connections (much of this is unknown even for mature granule cells). The study by Vivar is not entirely qualitative though, and does provide some numbers that are interesting food for thought.
The quantitative data are primarily ratios of upstream 'traced' neurons for every adult-born neuron that is contacted. They found that adult-born neurons receive inputs from about one (or fewer): CA3 pyramidal neurons, mature granule cells, and septal neurons. The number was higher for cortical input neurons (~8), local inhibitory interneurons (~3) and excitatory hilar neurons (~5). In total, by 90 days of age, there were about 15 upstream cells for every adult-born neuron. So the majority of inputs come from the cortex, and local inhibitory and excitatory circuits. This is not entirely surprising but these numbers do seem a little low to me. Individual granule cells have thousands of synaptic inputs - shouldn’t thousands (or at least hundreds) of presynaptic neurons be labelled per starter cell? Does the low number reflect a maturation process that is much longer than widely believed? Or is the rabies virus not transmitted with 100% efficacy?
Final Words.
The big finding of this study is that immature neurons in the adult brain seem to wire up differently than their older, pre-existing counterparts. Even without functional analyses, the unique presence or absence of certain connections strongly suggests that the adult-born cells are doing something different in the brain. The less big finding of this study is that adult-born neurons receive some synaptic inputs that appear similar to what has previously been described for mature neurons. But nobody’s ever examined them. In several cases we still don’t even know what they are doing to the mature neurons. I think seeing that they exist reminds us about all that we do not know, and need to investigate, in order to understand what immature and mature neurons are doing in the adult brain.
References
Vivar et al. (2012) Monosynaptic inputs to new neurons in the dentate gyrus. Nat Commun,3:1107
1Actually, the paper is ancient by blogging standards but it's an important one to cover.
2A more recent study has used a similar viral strategy and similarly found that mature granule neurons synapse onto adult-born neurons. But they concluded that this was caused by nonspecific infection of mature neurons by the retrovirus. So the mature to immature connection may need to be verified.
3Except this one.
4Alteratively, maybe adult-born neurons just take reaaaally long to develop these synapses, i.e. when they’re older than 90 days (oldest age examined). The other study also noted that entorhinal cortex neurons synapse onto adult-born neurons but unfortunately they didn’t specify medial vs. lateral subregions.
Also In Cells & Circuits
Trending
Popular articles on BrainFacts.org