Could Mitochondria Be the Key to a Healthy Brain?
- Published20 Jul 2021
- Author Diana Kwon
- Source Knowable Magazine
Long before the earliest animals swam through the water-covered surface of Earth’s ancient past, one of the most important encounters in the history of life took place. A primitive bacterium was engulfed by our oldest ancestor — a solo, free-floating cell. The two fused to form a mutually beneficial relationship that has lasted more than a billion years, with the latter providing a safe, comfortable home and the former becoming a powerhouse, fueling the processes necessary to maintain life.
That’s the best hypothesis to date for how the cellular components, or organelles, known as mitochondria came to be. Today, trillions of these bacterial descendants live within our bodies, churning out ATP, the molecular energy source that sustains our cells. Despite being inextricably integrated into the machinery of the human body, mitochondria also carry remnants of their bacterial past, such as their own set of DNA.
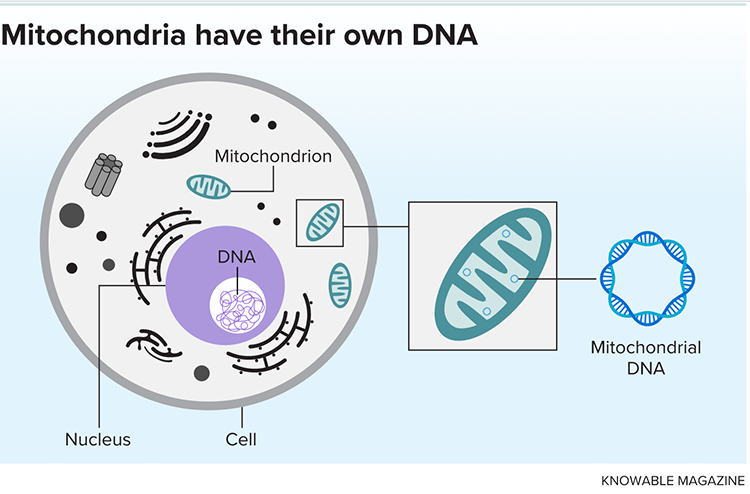
These features make mitochondria both a critical element of our cells and a potential source of problems. Like the DNA inside the nuclei of our cells that makes up the human genome, mitochondrial DNA can harbor mutations. Age, stress and other factors may disrupt mitochondria’s many functions. On top of that, mitochondrial injury can release molecules that, due to their similarities to those made by bacteria, can be mistaken by our immune system as foreign invaders, triggering a harmful inflammatory response against our own cells.
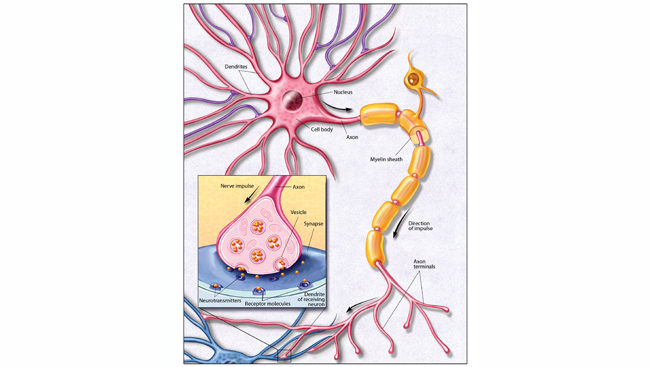
There is one organ that appears to be particularly vulnerable to mitochondrial damage: our power-hungry brains. “The more energetically demanding a cell is, the more mitochondria they have, and the more critical that mitochondria health is — so there’s more potential for things to go wrong,” says Andrew Moehlman, postdoctoral researcher who studies neurodegeneration at the US National Institute of Neurological Disorders and Stroke (NINDS). According to some estimates, each neuron can have up to 2 million mitochondria.
A small but growing number of scientists are now turning their attention to the contributions of mitochondria in brain health. Studies in humans and lab animals — though much of it still preliminary — suggest these organelles could be key players in virtually every type of brain disorder, including neurodevelopmental conditions such as autism, psychiatric illnesses like depression and schizophrenia, and neurodegenerative diseases such as Parkinson’s. They may even be at the heart of an enduring mystery for researchers who study brain disorders: how genetic predispositions and environmental influences interact to put people at risk for developing these conditions.
Problems at the powerhouse
In the 1960s, researchers discovered that mitochondria possess a unique set of genetic material. Investigations revealed that mitochondrial DNA, like that of bacteria, forms a circular strand and encodes just 37 genes — a mere fraction of the tens of thousands found in the human genome.
A short time later, in the 1970s, a doctoral student at Yale University named Douglas Wallace developed an interest in mitochondrial DNA. Wallace reasoned that since mitochondria were the primary producers of the body’s energy, mutations in their DNA would lead to disease. “At the time no one thought it was rational,” he says. It wasn’t until 1988, when Wallace and his colleagues established the first link between a mutation in mitochondrial DNA and a human disease — Leber’s hereditary optic neuropathy, a condition that causes sudden blindness — that medical researchers began to take the idea seriously, Wallace recalls.
Researchers have since linked dozens of disorders to alterations in mitochondrial DNA and nuclear DNA related to mitochondrial function — and interestingly, the majority of these are either neurological in nature or have some effect on the brain. Wallace, who is now director of the Children’s Hospital of Philadelphia’s Center for Mitochondrial and Epigenomic Medicine, has a simple explanation: Despite making up only 2 percent of a human’s body weight, the brain uses roughly a fifth of the body’s energy. In the same way that high-energy appliances will be disproportionately affected when voltage levels drop during a metropolitan brownout, even small reductions in mitochondrial function can have large effects on the brain, Wallace says.
Wallace is particularly interested in how mitochondria might contribute to autism spectrum disorder. Studies by several research teams have revealed that mitochondrial diseases, a mix of symptoms caused by defects in the organelle, are much more prevalent in people with autism (5 percent) than in the general population (about 0.01 percent). An additional 30 percent to 50 percent of children with autism show signs of mitochondrial dysfunction, such as abnormal levels of certain byproducts generated by cellular respiration, the process through which ATP is produced.
In some people with autism, scientists have identified genetic differences either in mitochondrial DNA, or in some of the thousand or so genes in the human genome known to influence mitochondrial function. More work is needed to establish whether these genetic variations actually cause or contribute to autism, but a recent study with mice hints that there could be a link. Wallace and colleagues reported earlier this year in PNAS that a specific mutation in mitochondrial DNA can lead to autism-like traits in mice, including impaired social interactions, skittishness and compulsive behavior.
Genetic alterations aren’t the only way mitochondria could contribute to autism. Certain environmental factors, such as toxic pollutants, have been associated with a higher risk of developing the condition. Richard Frye, a pediatric neurologist and autism researcher at the Phoenix Children’s Hospital in Arizona, and his colleagues have found that such factors may also perturb the health of mitochondria in people with autism. In one study, they found that the amount of air pollution that children with autism were exposed to before birth altered the rates at which their mitochondria produced ATP. In another, the researchers found correlations between early-life exposure to both nutritional metals such as zinc as well as toxic metals such as lead, and how well the organelles functioned in those with autism later in life. Together, Frye says, these findings suggest that mitochondria be the missing link between autism and the environmental influences that contribute to the condition.
“It’s too soon to make any firm conclusions about a lot of this stuff, but it sure looks like the mitochondria are disrupted in many kids with autism,” Frye says. “And environmental exposures, especially early on, may be programming the mitochondria to have different types of respiratory physiology.”
Researchers have also found signs of mitochondrial dysfunction, such as disturbances in the way they metabolize sugars to create energy, in people with schizophrenia and depression. In addition, studies also suggest that mitochondria may be sensitive to a risk factor for many mental illnesses: psychological stress in early life. For example, people who experience a traumatic event in childhood appear to have a larger number of mitochondrial genomes per cell. This uptick in mitochondrial DNA — which can indicate the formation of new mitochondria — may occur to compensate for problems in the organelle, according to Teresa Daniels, a biological psychiatry researcher at Brown University, where she is working on addressing this question. Daniels is a coauthor of a 2020 paper in the Annual Review of Clinical Psychology that discusses the role of mitochondria in psychiatric disorders.
Although mitochondrial dysfunction appears in a wide range of brain disorders, it’s not yet clear whether defects in these organelles are a primary cause of these conditions or a secondary effect, says Robert McCullumsmith, a physician-scientist at the University of Toledo who studies brain disorders but is not involved in the work on mitochondria. “It’s a bit of a chicken-and-egg problem,” he says. However, McCullumsmith adds, studying the role of mitochondria in these disorders is important, and he sees promising evidence that therapeutics that target mitochondria may end up benefiting patients, even if they don’t cure these conditions.
When friend becomes foe
When mitochondria become damaged or dysfunctional, one consequence is simply less ATP, and therefore less energy for the normal operations of the brain. But another way mitochondria could contribute to brain disorders stems from their ancestral past.
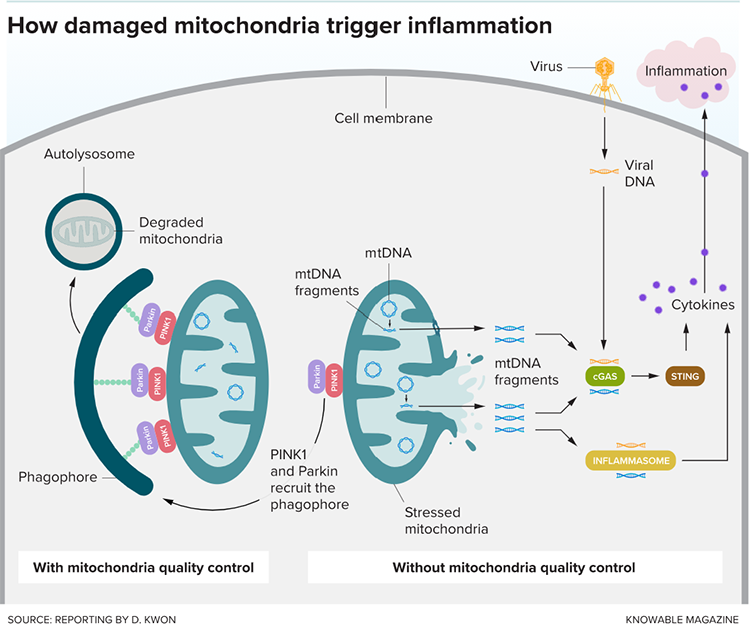
As descendants of bacteria, mitochondria have DNA and other components that can be released when cells are injured or stressed and mistaken by our immune system as a foreign threat. In 2010, researchers at Harvard University reported a rapid release of mitochondrial DNA into the bloodstream in people with severe physical injuries — such as fractures or hemorrhages caused by a car crash. This, in turn, attracted immune cells and triggered a severe inflammatory response that mimicked sepsis — a life-threatening condition in which the immune system attacks the body’s own tissues.
A few years later, A. Phillip West, who was then a postdoc at Yale University, and his colleagues showed that DNA can leak out of mitochondria and activate the immune system even in the absence of such severe injuries — for example, when the organelles became deficient in a key protein.
Inflammation caused by the release of mitochondrial DNA may contribute to the damage found in neurodegenerative diseases such as Parkinson’s, Alzheimer’s and amyotrophic lateral sclerosis (ALS), according to a growing number of studies. In separate lines of research, scientists have linked these disorders with both inflammation and an inability to properly rid cells of defective mitochondria. Mitochondria-triggered inflammation may be the missing link between the two.
For example, mutations in two genes associated with some forms of inherited Parkinson’s disease — PINK1 and PRKN — lead to problems in the process through which damaged mitochondria are broken down and cleared from the cell. In 2019, a group led by Richard Youle at the NINDS demonstrated that in mice with mutations in PINK1 and PRKN, inducing mitochondrial damage (either through exhaustive exercise or by altering mitochondrial DNA) activated inflammatory molecules. Those animals also lost dopamine-producing neurons in their brains and developed problems with movement — hallmarks of Parkinson’s disease. These effects didn’t occur, however, when the researchers repeated the experiment with mice engineered to lack an important inflammatory molecule. Together, these findings illustrated that in animals genetically predisposed to Parkinson’s, either stress or glitches in mitochondrial DNA could trigger the inflammation that promotes the disease.
Although more work is needed to establish whether the same process occurs in humans, “there’s a lot of evidence that the failure to maintain the healthy mitochondria is one of the early pathological events that leads to development of Parkinson’s symptoms,” says Moehlman, who coauthored a 2020 paper in the Annual Review of Cell and Developmental Biology with Youle that discusses how problems in mitochondria may lead to neurodegeneration.
As evidence mounts that leaking mitochondrial DNA is bad news, some researchers are turning their attention to why. Many processes may be at play, says West, who is now an immunobiologist Texas A&M University. One scenario, he says, is that the organelle ejects constant, low levels of DNA over time — and when exacerbated by genetic or environmental factors, this accumulation can reach a threshold where diseases occur.
Psychological stress could be one such factor. In a 2019 study, Martin Picard, a mitochondrial psychobiologist at Columbia University, and his colleagues reported that after a brief public-speaking task where participants were asked to defend themselves against an alleged transgression, levels of free-floating mitochondrial DNA in the bloodstream rose, indicating that the mitochondria had expelled their genetic material.
This sort of mitochondrial damage and DNA release could contribute to human diseases where inflammation appears to play a role, even in the absence of an infection, such as cancer, autoimmune conditions and neurodegenerative disorders, West says.
He and others also suspect that mitochondria-induced inflammation may be a key driver of aging itself. In a recent study, West’s team demonstrated that mice engineered to have unstable mitochondrial DNA aged more quickly, developing problems such as hair and bone loss and dying prematurely. Eliminating the elements of the immune system activated by mitochondria DNA reversed this process, extending the animals’ lifespans by around 40 days. (These results were posted before publication on bioRxiv and have yet to be peer-reviewed.) If future research bears this out, it would provide evidence that aging, in these mice at least, is partly driven by mitochondrial damage, West says.
Multipurpose mitochondria
Mitochondria have other functions that help maintain healthy brain function — or cause problems when they go awry. For example, mitochondria help control the balance of potentially toxic byproducts of cellular metabolism called reactive oxygen species and the synthesis of stress hormones like cortisol. Mitochondria are also highly dynamic — communicating with each other via signaling molecules and physical connections. They continuously undergo fission, where a large mitochondrion splits into two smaller ones, or fusion, when they combine. These ongoing interactions may also influence brain function and behavior in ways that researchers are only beginning to realize.
Carmen Sandi, a behavioral neuroscientist at the Swiss Federal Institute of Technology, and her group have examined mitochondria in mice with high levels of anxiety-like behaviors, such as less willingness to spend time in open areas. They’ve found that in those animals, mitochondria in the neurons of the nucleus accumbens, a brain area involved in processing reward, were less adept at producing ATP compared to those found in animals that displayed lower levels of anxiety. The high-anxiety animals also displayed lower levels of an enzyme involved in fusion — which enables mitochondria to combine and mix their contents to support one another in times of need. Increasing the level of this protein not only restored mitochondrial function, but also reduced anxious behaviors, the researchers found.
Findings like these give scientists reason to hope they may one day be able to develop treatments for brain disorders that target these organelles. Frye, for example, recently began a clinical trial to investigate whether nutrient supplements can reverse the mitochondrial abnormalities his team has found in children with autism. Wallace adds that researchers already know of many potential treatments that help boost the function of mitochondria — from medications to behavioral interventions, such as exercise.
It will take time to test such interventions. For now, scientists are busy unraveling the multitude of functions mitochondria have in the brain. Much of this work is still preliminary, but evidence coming from a variety of disciplines — including neuroscience, immunology and psychology — has scientists excited about the future. There is plenty of room for new discoveries about mitochondria, says Sandi. “I think they are doing much more than what neuroscientists have believed in the past.”
CONTENT PROVIDED BY
Knowable Magazine is an independent journalistic endeavor from Annual Reviews.