The Roots of Autism
- Published12 Apr 2018
- Author Jennifer Michalowski
- Source BrainFacts/SfN

The Discovery
Genetic Connections
The stories can be devastatingly similar. A seemingly normal toddler stops talking and becomes withdrawn, missing important developmental milestones. The diagnosis: autism. Parents may blame themselves. For decades, the medical community did too, particularly blaming cold, aloof mothers as the cause.
In 1964, Bernard Rimland, a psychiatrist and father of an autistic boy, made a bold proposal — what if autism’s roots lie not with bad parenting, but with a fault in biology?
When scientists want to untangle the influence of environment from biology, they turn to twin studies. Identical twins possess the same genes, whereas fraternal twins share no more genes than any other pair of siblings. Genes play a role when a condition occurs in both members of a pair of identical twins far more often than in fraternal twins. In 1977, Susan Folstein and Michael Rutter, from London’s Institute of Psychiatry, studied 21 sets of twins, finding more than a third of the identical twins with autism shared the diagnosis with their twin. But, none of the fraternal twins with autism did. They found genes were playing an important role in autism, but it would be decades before researchers had the tools to find those genes.
Completed in 2003, the Human Genome Project took 13 years and cost over a billion dollars. Today’s DNA sequencing technologies allow researchers multiple ways to examine the human genome quickly and cheaply — roughly $1,000 or less for each genome. Using these technical advances, researchers including Matthew State at the University of California, San Francisco, Daniel Geschwind at the University of California, Los Angeles, and Michael Wigler at Cold Spring Harbor Laboratory analyzed the genomes of thousands of people, both with and without autism, identifying hundreds of genetic factors that seem related to the disorder. More than a dozen of those genes appear to strongly influence whether a person has autism, while the others have smaller impacts, working in concert to have their effect.
These genes provide important clues to the roots of autism; but, they fail to explain how brain development goes awry or what causes symptoms like repetitive handwringing.
A Critical Period
Prenatal Brain Development
Autism is different in every person with the disorder. Some children struggle to interact with others or manage emotions, some become intensely focused on a narrow range of interests, or exhibit extraordinary talents, while some cannot speak and have significant cognitive impairments. Parents and other caregivers usually start noticing something is amiss after a child’s first year.
While the brain continues to develop after birth, it is built before birth when neurons and other brain cells rapidly divide, migrate to new locations, and form their first connections with one another.
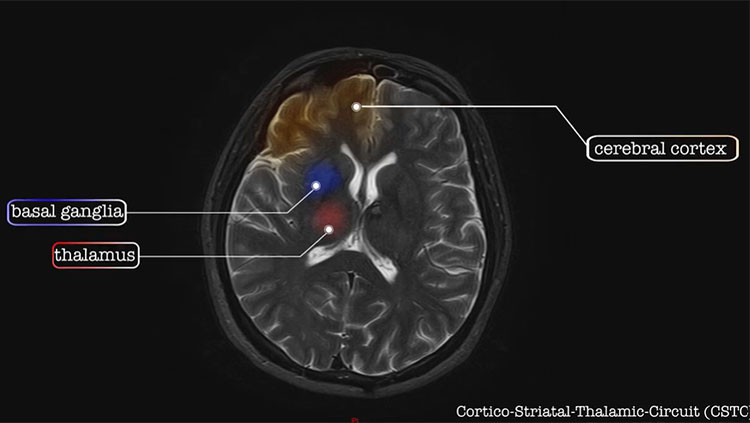
The process requires precise regulation, and evidence is building that it goes awry in people with autism. In 2010, Jerzi Wegiel’s team at the New York Institute for Basic Research examined postmortem brain tissue from people with autism finding disruptions in the way cells grew and moved through the developing brain. Similarly, Cynthia Shumann at the University of California, Davis, discovered people with autism possessed abnormal numbers of neurons in the amygdala, a central processor of emotional behaviors.
In order to understand what happens in the brain of an autistic person before birth, Emanuel DiCicco-Bloom’s team at Rutgers Robert Wood Johnson Medical School are studying how stem cells derived from the cells of children with autism develop into neurons.
Preliminary findings hint that for people with autism, neurons struggle to form the cellular outgrowths that eventually forge connections with other cells. Experiments in mice indicate autism-like behaviors are prevalent when neurons fail to trim back the communication-mediating protrusions called dendritic spines.
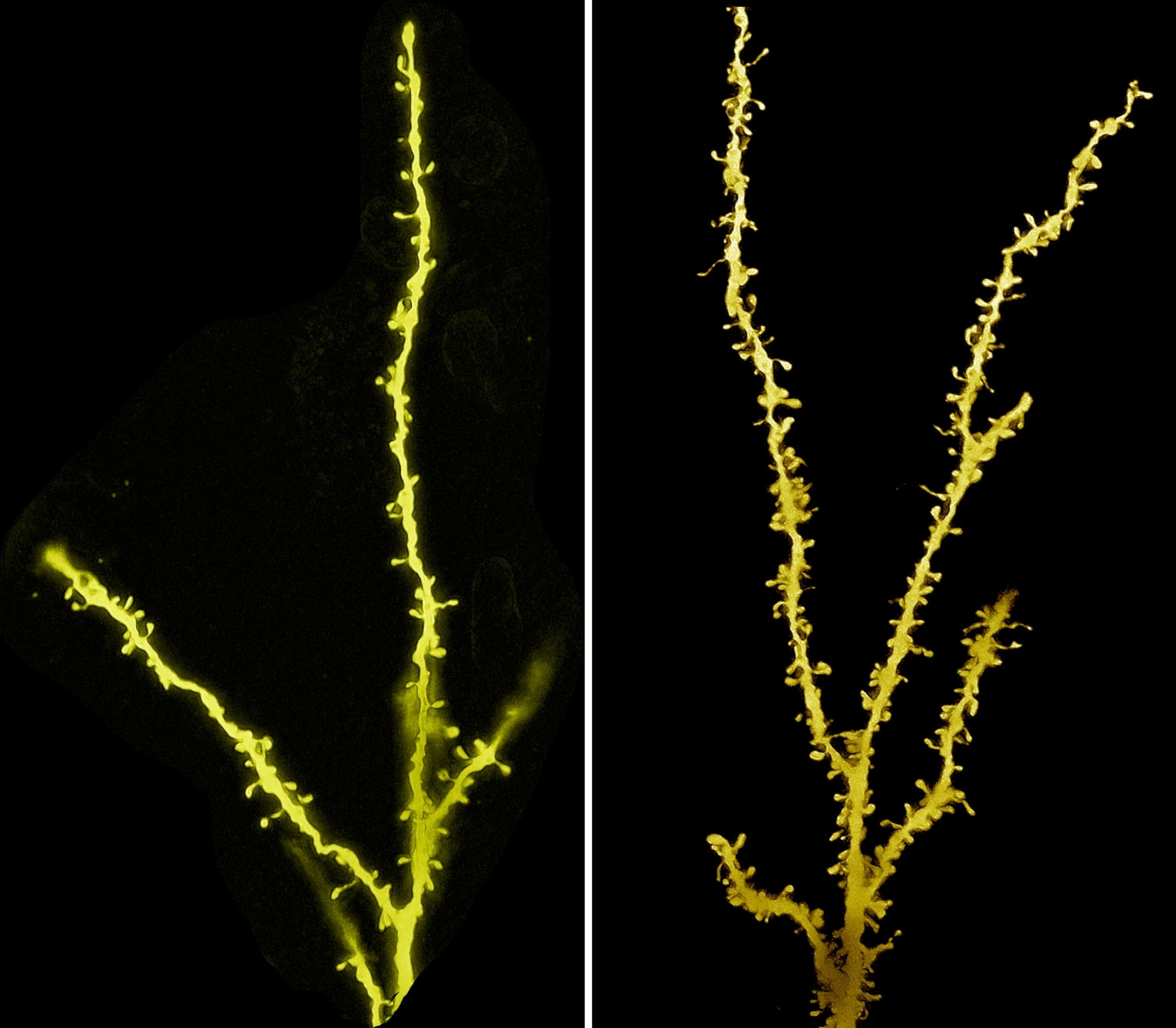
With numerous genes linked to autism, the disorder’s characteristic behavior patterns likely arise from many different disruptions to brain development. By looking at networks of hundreds of autism genes, Geschwind’s UCLA team pinpointed a number involved in the prenatal development of the brain’s cortex. Researchers led by UCSF’s Matthew State, then at Yale University, focused on nine strongly linked autism genes. By identifying networks of genes that are active at the same time and place in the brain, State’s team found the window in which cortical development may be disrupted: during the first or second trimester of pregnancy.
Pathways to Intervention
Personalized Approaches
As they uncover the causes and consequences of the developmental problems associated with autism, researchers are hopeful that what they learn will lead to better diagnoses and treatments.
Currently, doctors diagnose autism based on behavioral patterns that don’t become apparent until a child’s second year of life. Identifying structural or functional features of the brain that precede these behaviors could allow them to implement interventions sooner. That’s important, because studies of infants considered to be at high risk of developing autism suggest that early intervention improves outcomes.
The early developmental problems associated with autism likely have wider impacts than the behavioral ones most commonly associated with the disorder. People with autism commonly experience problems with sensory processing. Researchers — including Stewart Mostofsky at the Kennedy Krieger Institute — have also found that the brain’s motor circuits work differently in people with the disorder. Monitoring these functions early in life may aid in early diagnosis.
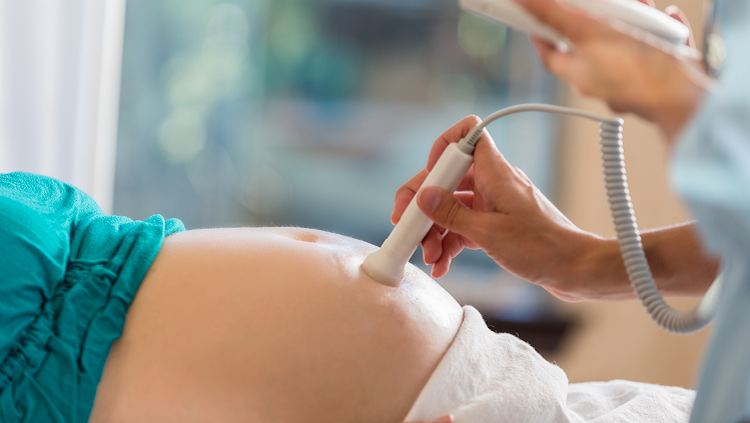
Earlier diagnosis may be possible. A 2017 study by Joseph Piven and colleagues at the University of North Carolina at Chapel Hill hints at that. They visualized brain activity in the infant siblings of children with autism using functional MRI scanning. Eleven of the 59 infants in their study were later diagnosed with autism. The brain scans taken when the infants were just six months old predicted nine of these diagnoses.
A better understanding of the neural networks and developmental anomalies underlying autism could also pave the way toward more precise diagnoses and personalized treatment. Behavioral interventions can improve language and social skills for many people with autism, and eventually, as researchers deepen their understanding of autism’s causes, they may be able to develop molecular therapies to ameliorate or reverse their effects on the brain.
Continued research funding from public and private institutions may allow scientists to identify autism spectrum disorder early, and to intervene based on the underlying biology affecting each individual to give people with autism the best opportunity to overcome the social, cognitive, and behavioral challenges associated with autism.
CONTENT PROVIDED BY
BrainFacts/SfN
Discussion Questions
(1) What myth did scientists debunk regarding the connection between parenting and autism?
(2) Name an animal model that researchers use to study the link between genes and autism.
(3) How do doctors currently diagnose autism?
References
Avino TA, Barger N, Vargas MV, Carlson EL, Amaral DG, Bauman MD, Schumann CM. Neuron numbers increase in the human amygdala from birth to adulthood, but not in autism.
Proc Natl Acad Sci U S A. 2018 Mar 20. pii: 201801912. doi: 10.1073/pnas.1801912115.
Bourgeron T. Current knowledge on the genetics of autism and propositions for future research. C R Biol. 2016;339(7-8):300-7.
De la torre-ubieta L, Won H, Stein JL, Geschwind DH. Advancing the understanding of autism disease mechanisms through genetics. Nat Med. 2016;22(4):345-61.
Emerson RW, Adams C, Nishino T, et al. Functional neuroimaging of high-risk 6-month-old infants predicts a diagnosis of autism at 24 months of age. Sci Transl Med. 2017;9(393).
Folstein, S. and Rutter, M. (1977), Infantile autism: a genetic study of 21 twin pairs.
Journal of Child Psychology and Psychiatry, 18: 297–321.
Gaugler T, Klei L, Sanders SJ, et al. Most genetic risk for autism resides with common variation. Nat Genet. 2014;46(8):881-5.
Marko MK, Crocetti D, Hulst T, Donchin O, Shadmehr R, Mostofsky SH. Behavioural and neural basis of anomalous motor learning in children with autism. Brain. 2015;138(Pt 3):784-97.
Parikshak NN, Luo R, Zhang A, et al. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell. 2013;155(5):1008-21.
Sebat J, Lakshmi B, Malhotra D, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316(5823):445-9.
Shen MD, Piven J. Brain and behavior development in autism from birth through infancy. Dialogues Clin Neurosci. 2017;19(4):325-333.
Tang G, Gudsnuk K, Kuo SH, et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron. 2014;83(5):1131-43.
Webb SJ, Jones EJ, Kelly J, Dawson G. The motivation for very early intervention for infants at high risk for autism spectrum disorders. Int J Speech Lang Pathol. 2014;16(1):36-42.
Wegiel J, Kuchna I, Nowicki K, et al. The neuropathology of autism: defects of neurogenesis and neuronal migration, and dysplastic changes. Acta Neuropathol. 2010;119(6):755-70.
Williams M, Prem S, Zhou X, Matteson P, Yeung PL, Lu CW, Pang Z, Brzustowicz L, Millonig JH, Dicicco-Bloom E. Rapid Detection of Neurodevelopmental Phenotypes in Human Neural Precursor Cells (NPCs).J Vis Exp. 2018 Mar 2;(133). doi: 10.3791/56628. PMID:29553565
Willsey AJ, Sanders SJ, Li M, et al. Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of autism. Cell. 2013;155(5):997-1007.
https://www.genome.gov/images/content/costpergenome_2017.jpg