A Rare Salamander Could Change How We Treat Spinal Cord Injuries
- Published4 Apr 2022
- Author Calli McMurray
- Source BrainFacts/SfN

A crown of fluffy gills pokes above the gravel, swaying with the movement of the water. Its owner, a pale pink salamander, scrounges around the bottom of Mexico’s Lake Xochimilco, looking for small fish and mollusks to eat. The axolotl inhales its prey — until danger strikes. A tilapia charges toward the axolotl and chomps off its front leg before the axolotl can escape.
But the axolotl’s loss will not be permanent. Within a day, new skin has grown over the wound. Within weeks, the arm is back. Tweak the type of injury, and you will see the same result: axolotls can regrow their tail, lungs, liver, spinal cord, heart, teeth, and even their brain.
Understanding the remarkable regenerative ability of the axolotl could mark a new chapter in human healing. It is unlikely humans will ever be able to grow back as many body parts as the axolotl, yet lessons from the salamander may bolster ongoing spinal cord injury treatments.
Going Back to the Start
When the human body is wounded, “Cells react to the injury, but instead of generating a regenerative response, they go down the path of scarring,” says James Monaghan, an associate professor in biology at Northeastern University. “We just patch up the injury and call it a day.” Thick scar tissue protects the wound from further damage, Monaghan says, but it also prevents cells from instigating regeneration.
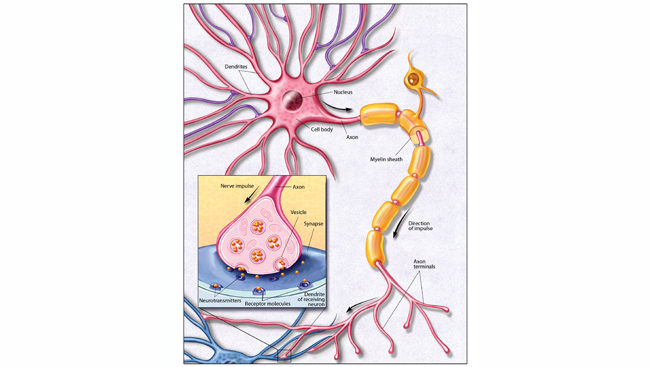
In the axolotl, however, a wound sets off a chain reaction. The epithelium — the top layer of skin — immediately begins to grow over the open wound, a process that takes days for humans. Soon after it closes, cells near the injury site migrate to the wound, like the connective tissue cells called fibroblasts. “They all come together to make this heterogeneous structure called a blastema,” Monaghan says. “Those cells within that blastema then know what's missing and can turn back time to a more developmental state.” These cells are not stem cells: they are mature, specialized cells that can somehow shed their programming and become blank slates again. Once this happens through a process called dedifferentiation, the cells can then re-differentiate into whatever type of cell is needed to regrow the limb (or liver, or spinal cord).
I think the cool thing is that there's no special cell type in the axolotl that humans, or mice, or other mammals don't have,” says Katharina Lust, a postdoc at the Research Institute of Molecular Pathology in Vienna, Austria. “But [regeneration is driven by] these cells that we also have, and they do something special, which cells in a mammalian limb don't do.”
The axolotl does not appear to have special regenerative genes, either. Scientists in Elly Tanaka’s lab — where Lust is completing her postdoc — sequenced the entire axolotl genome for the first time in 2018. The list of genetic information is very long, ten times longer than the human genome. Still, most of the length comes from repeated sequences, not unique genes. “Most of the genes we have, they also have,” Monaghan says. “It's not that there might be unique salamander genes that participate in regeneration, but all animals generated limbs originally.” Axolotls just re-access the genes humans only use while they develop during pregnancy.
Now that their entire genome has been cataloged, scientists like Monaghan and Lust can perform nitty-gritty molecular studies to zoom in on the exact proteins and processes driving regeneration. These types of studies involve turning individual genes on and off to see how each impacts regeneration and changing the recipe for the chemical stew of growth factors and proteins present at the wound site.
Setting up this local environment may be as critical as manipulating the right genes. “It's not as easy as saying, 'We know gene X, Y, Z,' and we can put these genes into these mouse fibroblasts and everything will work perfectly,” Lust says. “There are also environmental factors.”
Monaghan studies one such factor called neuregulin, which is secreted by nerves and is involved in cell growth and division, also known as proliferation. In one study, “We were able to block the signaling pathway of neuregulin, which blocked regeneration,” Monaghan says. “Then, we could get rid of nerves and supplement the limb with extra neuregulin, [which] increased self-proliferation and partially rescued the regeneration without nerves."
A Path to Human Healing
Even before scientists crack the genetic code of regeneration, axolotl studies can support ongoing research into treatments for human spinal cord injuries. Grégoire Courtine, a professor at the Swiss Federal Institute of Technology Lausanne, and his team have developed one such therapy involving electrical stimulation.
When the spinal cord is damaged, signals from the brain cannot travel through the injury, and signals below the injury cannot travel up to the brain. Yet even “in the majority of people with complete paralysis, there are some residual connections that are silent: anatomically intact, but functionally silent,” Courtine says. “When you stimulate the lumbar spinal cord below the injury, [these connections] become functional.” In less severe injuries, a zap of electricity enables people to initiate a step just by thinking about it. In more severe cases, “They use clickers. So they click, and pewf: they start stepping,” Courtine says, referencing the speed at which patients start moving from the electrical stimulation. “And if they don't click, they stand.”
This treatment, called neuromodulation, can improve quality of life by increasing mobility and independence but does not heal the spinal cord. “We must combine neuromodulation with what I like to call spinal cord repair intervention, because the neuromodulation is not enough,” Courtine says. “I believe it will become a treatment to support people, help them recover better, empower them basically to recover more than they would without stimulation, but it is still not a cure for spinal cord injury.”
These repair strategies involve transplanting stem cells to the site of the injury, which Courtine is currently studying in rats. That is where the axolotl can be of service: “Stem cell therapies for spinal cords have moved a long way,” Monaghan says, “and they're showing really promising positive results. But these stem cell therapies might be enhanced by mimicking the environment that the axolotl uses to regenerate its spinal cord.” Injecting growth factors like neuregulin alongside the stem cells could nudge regeneration forward. Even if humans can never grow back a limb, axolotl research holds potential to improve the way we heal.
CONTENT PROVIDED BY
BrainFacts/SfN
References
Axolotl: National geographic. Animals. (n.d.). Retrieved March 14, 2022, from https://www.nationalgeographic.com/animals/amphibians/facts/axolotl
Bassat, E., & Tanaka, E. M. (2021). The cellular and signaling dynamics of salamander limb regeneration. Current opinion in cell biology, 73, 117–123. https://doi.org/10.1016/j.ceb.2021.07.010
Farkas, J. E., Freitas, P. D., Bryant, D. M., Whited, J. L., & Monaghan, J. R. (2016). Neuregulin-1 signaling is essential for nerve-dependent axolotl limb regeneration. Development (Cambridge, England), 143(15), 2724–2731. https://doi.org/10.1242/dev.133363
Nowoshilow, S., Schloissnig, S., Fei, J.-F., Dahl, A., Pang, A. W., Pippel, M., Winkler, S., Hastie, A. R., Young, G., Roscito, J. G., Falcon, F., Knapp, D., Powell, S., Cruz, A., Cao, H., Habermann, B., Hiller, M., Tanaka, E. M., & Myers, E. W. (2018). The axolotl genome and the evolution of key tissue formation regulators. Nature, 554(7690), 50–55. https://doi.org/10.1038/nature25458
Rowald, A., Komi, S., Demesmaeker, R., Baaklini, E., Hernandez-Charpak, S. D., Paoles, E., Montanaro, H., Cassara, A., Becce, F., Lloyd, B., Newton, T., Ravier, J., Kinany, N., D’Ercole, M., Paley, A., Hankov, N., Varescon, C., McCracken, L., Vat, M., … Courtine, G. (2022). Activity-dependent spinal cord neuromodulation rapidly restores trunk and leg motor functions after complete paralysis. Nature Medicine, 28(2), 260–271. https://doi.org/10.1038/s41591-021-01663-5
Squair, J. W., Gautier, M., Sofroniew, M. V., Courtine, G., & Anderson, M. A. (2021). Engineering spinal cord repair. Current opinion in biotechnology, 72, 48–53. https://doi.org/10.1016/j.copbio.2021.10.006