How Ketamine Changed Our Understanding of Depression and Mental Health
- Published15 Oct 2020
- Author Levi Gadye
- Source BrainFacts/SfN
When looking for the neural basis of mood disorders, scientists zeroed in on the mood centers of the brain and the neurotransmitters regulating them. But studies of the anesthetic and club drug ketamine shifted this focus. They suggested depression caused disruptions in brain circuits, rather than a singular defect in one structure or neurotransmitter system.
A Transformative Effect
In the late 1990s, John Krystal initiated a small study on ketamine in humans with modest expectations. Intrigued by research in mice, the Yale psychiatrist posited ketamine’s effects could help depressed people.
In mice, the anesthetic and club drug ketamine blocks glutamate receptors in several brain regions responsible for higher-level functions like reasoning, planning, and decision-making. Drugs with similar effects appeared to ameliorate symptoms in animal models of depression.
So Krystal and colleague Dennis Charney gave seven depressed patients a single, low dose of ketamine and tracked their mood for two weeks. The effects were remarkable — and immediate.
Four hours after receiving ketamine, well after the drug’s intoxicating effects had worn off, the patients “began to say things like, ‘I feel a little bit lighter, a little bit more relaxed, a little clearer,’” Krystal recalls. “The next morning, we actually had some people who said, ‘I feel like I’m back to myself.’”
No antidepressant had ever alleviated depression so quickly. Available antidepressants required over a month of daily doses before patients would see an improvement. Krystal and his colleagues were understandably skeptical of what they were witnessing.
“We really didn’t think that much of it until we had several people who had, within 24 hours of a single dose of ketamine, marked improvement of their clinical condition,” he says. “It had a transformative effect on my view of the biology of depression.”
When looking for the neural basis of mood disorders, scientists zeroed in on the mood centers of the brain, collectively called the limbic system. But that was only part of the story. The small pilot study — and years of follow-up — ushered in a paradigm shift for depression. Irrespective of whether ketamine is a suitable therapy for common depression, it suggested depression was caused by disruptions in brain circuits, rather than a singular defect in one structure or neurotransmitter system.
Beyond the Monoamine Hypothesis of Depression
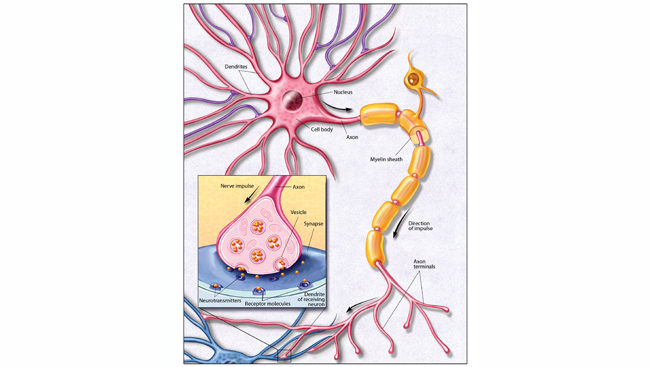
Our most basic emotions, like fear, anger, and disgust, arise deep within the brain in a set of interconnected regions called the limbic system. Making sense of and regulating these emotions is the domain of the prefrontal cortex, which also handles complex cognitive abilities, like decision-making.
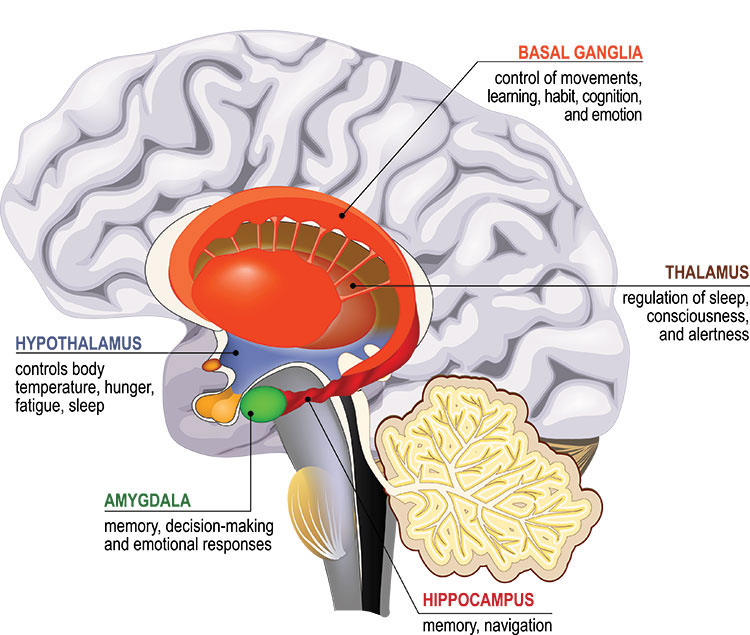
Nerve cells deep within the brain affect mood by releasing chemical messengers in the limbic system. In the 1960s and 70s, scientists discovered the available antidepressants elevated levels of these chemicals, called monoamines. Depression must result from low levels of monoamines, like serotonin.
However, boosting monoamines doesn’t help all depressed patients. And there’s little evidence they have lower levels of serotonin to begin with. By the late 1990s, Krystal maintains, a “conceptual crisis” for the monoamine hypothesis of depression took hold.
Were scientists missing a piece of the puzzle by focusing so narrowly on the monoamines?
In their first study of ketamine in depressed people, Krystal and Charney hoped to observe whether ketamine had any effect on depression symptoms. Ketamine instead quickly and effectively treated depression.
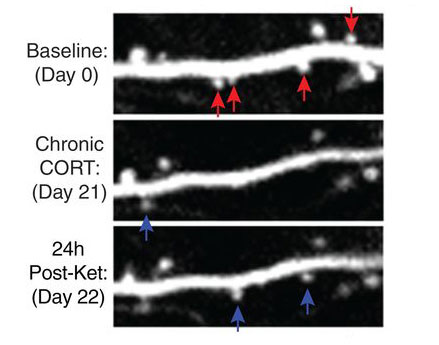
Over the next two decades, the field’s understanding of depression and emotional regulation — writ large — shifted. Randomized clinical trials led by Carlos Zarate at the National Institutes of Mental Health confirmed ketamine produced robust and rapid antidepressant effects. Scientists learned that stress — a known cause of depression — depleted synapses in the prefrontal cortex and limbic system. Yale’s Ronald Duman and others found ketamine spurred those synapses to grow back. In doing so, it seemed to rewire the brain to exert better control over negative emotions. These findings collectively lent themselves to a new hypothesis of depression, Krystal says, one recognizing the importance of the neural highways linking the prefrontal cortex to the limbic system.
While scientists figured out the “how” behind ketamine’s antidepressant effects, Krystal and Charney started exploring whether ketamine could help people with other mental illnesses. In 2014, Charney, along with colleague Adriana Feder, found ketamine improved symptoms in people with post-traumatic stress disorder (PTSD).
Strengthening the Connections That Matter
In 2019, the U.S. Food and Drug Administration approved a nasal-spray formulation of esketamine — a chemical cousin of ketamine — for people with severe, treatment-resistant depression. (Krystal, Charney, and Zarate are co-inventors of a patent licensed to Janssen Pharmaceuticals, the developers of the nasal spray.) Earlier this year, the FDA added approval for patients with suicidal thoughts or behavior.
“Ketamine is a lifesaver for people who are severely depressed and have not responded well to other antidepressants,” says Conor Liston, a psychiatrist and researcher at Weill Cornell Medicine. Liston’s work in animals indicates ketamine’s long-lasting effect derives from the growth of new synapses, which he believes could be bolstered by behavioral therapies.
A fast-acting drug with long-lasting effects could be a game changer for treating PTSD.
Humans have an exceptional knack for distinguishing true threats from false alarms, a necessity in the modern world. But for people with PTSD, the loud banging of a backfiring car can evoke disturbing memories and a heightened, or even inappropriate, emotional response, making it hard to live a normal life.
Overcoming traumatic memories requires patients to confront the memory and find new ways to address it. Ketamine could aid the process by facilitating the growth of new synapses.
“PTSD and depression share some symptoms and share some risk genetically,” and antidepressants are commonly used for PTSD, says Liston. “So, it stands to reason that a drug like ketamine, which has been so, so effective for some patients with depression, might also be useful in PTSD.”
Krystal is exploring ketamine’s potential in military personnel with PTSD. He’s wrapping up a large study with around 150 patients, one-third of whom are active-duty military.
Krystal and Liston look forward to seeing ketamine gain wider acceptance both as a clinical therapy for PTSD and as inspiration into new research on the role of the prefrontal cortex in mood disorders. Ketamine is not without drawbacks: it can dramatically alter perception and carries the potential for abuse. That’s why esketamine must be administered under a doctor’s supervision. It remains to be seen whether repeated administrations pose any additional safety concerns.
Ketamine hints at many novel treatments for mental health disorders, Krystal says. While it will take a long time to sort them all out, he’s optimistic. “Ketamine is the tip of the iceberg.”
CONTENT PROVIDED BY
BrainFacts/SfN
Discussion Questions
- What was the monoamine hypothesis of depression?
- How did findings from ketamine research challenge the monoamine hypothesis?
- How is ketamine thought to help people with severe depression or PTSD?
References
Berman, R. M., Cappiello, A., Anand, A., Oren, D. A., Heninger, G. R., Charney, D. S., & Krystal, J. H. (2000). Antidepressant effects of ketamine in depressed patients. Biological Psychiatry, 47(4), 351–354. https://doi.org/10.1016/S0006-3223(99)00230-9
Deyama, S., & Duman, R. S. (2020). Neurotrophic mechanisms underlying the rapid and sustained antidepressant actions of ketamine. Pharmacology, Biochemistry, and Behavior, 188, 172837. https://doi.org/10.1016/j.pbb.2019.172837
Duman, C. H., & Duman, R. S. (2015). Spine synapse remodeling in the pathophysiology and treatment of depression. Neuroscience Letters, 601, 20–29. https://doi.org/10.1016/j.neulet.2015.01.022
Feder, A., Parides, M. K., Murrough, J. W., Perez, A. M., Morgan, J. E., Saxena, S., Kirkwood, K., aan het Rot, M., Lapidus, K. A. B., Wan, L.-B., Iosifescu, D., & Charney, D. S. (2014). Efficacy of Intravenous Ketamine for Treatment of Chronic Posttraumatic Stress Disorder: A Randomized Clinical Trial. JAMA Psychiatry, 71(6), 681. https://doi.org/10.1001/jamapsychiatry.2014.62
Hare, B. D., & Duman, R. S. (2020). Prefrontal cortex circuits in depression and anxiety: Contribution of discrete neuronal populations and target regions. Molecular Psychiatry. https://doi.org/10.1038/s41380-020-0685-9
Krystal, J. H., & Roache, J. D. (n.d.). CAP-Ketamine for antidepressant resistant PTSD. Grantome. Retrieved October 14, 2020, from https://grantome.com/grant/NIH/I01-CX001142-05
Li, N., Lee, B., Liu, R.-J., Banasr, M., Dwyer, J. M., Iwata, M., Li, X.-Y., Aghajanian, G., & Duman, R. S. (2010). MTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science (New York, N.Y.), 329(5994), 959–964. https://doi.org/10.1126/science.1190287
Li, N., Liu, R.-J., Dwyer, J. M., Banasr, M., Lee, B., Son, H., Li, X.-Y., Aghajanian, G., & Duman, R. S. (2011). Glutamate NMDA receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biological Psychiatry, 69(8), 754–761. https://doi.org/10.1016/j.biopsych.2010.12.015
Moda-Sava, R. N., Murdock, M. H., Parekh, P. K., Fetcho, R. N., Huang, B. S., Huynh, T. N., Witztum, J., Shaver, D. C., Rosenthal, D. L., Alway, E. J., Lopez, K., Meng, Y., Nellissen, L., Grosenick, L., Milner, T. A., Deisseroth,K., Bito, H., Kasai, H., & Liston, C. (2019). Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science, 364(6436). https://doi.org/10.1126/science.aat8078
Trullas, R., & Skolnick, P. (1990). Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. European Journal of Pharmacology, 185(1), 1–10. https://doi.org/10.1016/0014-2999(90)90204-J
Zarate, C. A., Singh, J. B., Carlson, P. J., Brutsche, N. E., Ameli, R., Luckenbaugh, D. A., Charney, D. S., & Manji, H. K. (2006). A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Archives of General Psychiatry, 63(8), 856–864. https://doi.org/10.1001/archpsyc.63.8.856