The Tools That Let Neuroscientists Study (and Even Repair) Brain Circuits
- Published13 Feb 2018
- Reviewed13 Feb 2018
- Author Levi Gadye
- Source BrainFacts/SfN
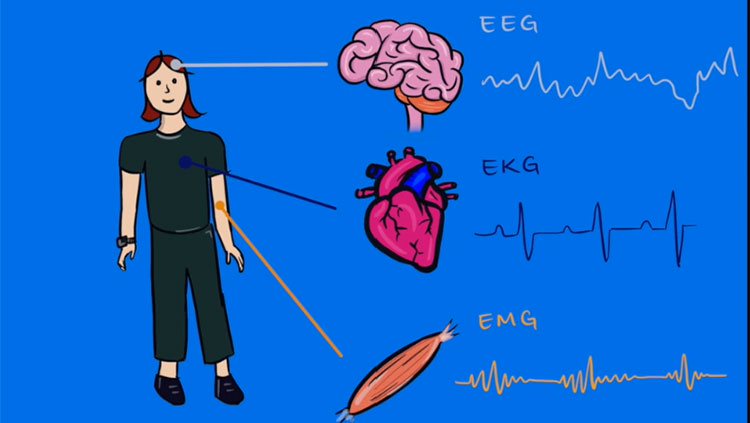
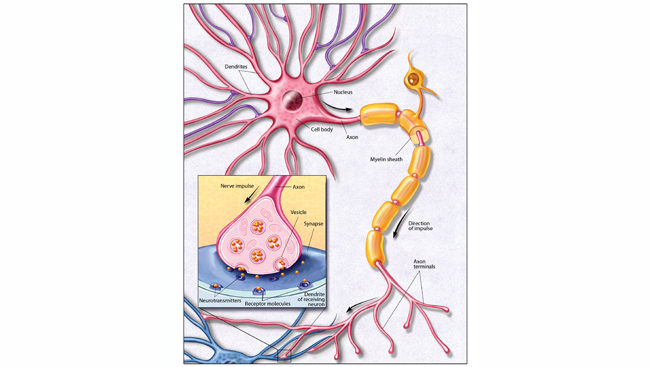
Neurons may be the building blocks of the brain, but no neuron works alone. Instead, neurons connect up with each other — like wires — into neural circuits and work together to accomplish certain tasks. Depending on how complex the task, some circuits contain only a few neurons, while others contain millions.
All neural circuits receive information, process and interpret it, and send out updated signals for the brain to use in response. For example, neural circuits in the eye convert light into information the brain can understand. Specialized cells called rods and cones detect light and transmit signals to neurons in the retina. These neurons collaborate to make sense of those signals, and then relay information about contrast, color, and motion to the visual centers of the brain.
Sometimes, though, neural circuits malfunction. Injury and disease can damage neurons, disrupting neural circuits — just like a tree falling on electrical wiring. Neural circuits can also go haywire if individual neurons become overactive or not active enough. Autism, epilepsy, and depression all result from problems in neural circuits.
With support from programs like the BRAIN Initiative, neuroscientists are inching closer to understanding how neural circuits work, and what can be done to fix malfunctioning circuits. Functional magnetic resonance imaging (fMRI) lets scientists take pictures of the brain to watch how human neural circuits work in real time. Transcranial magnetic stimulation (TMS) allows doctors to stimulate neural circuits in psychiatric patients to alleviate their symptoms. Advances in genetic engineering have even led to technologies that enable scientists to control neural circuits using light. The brain harbors many mysteries still, but we are better armed to solve them than ever before. Let’s take a closer look at the technologies that are spurring these advances.
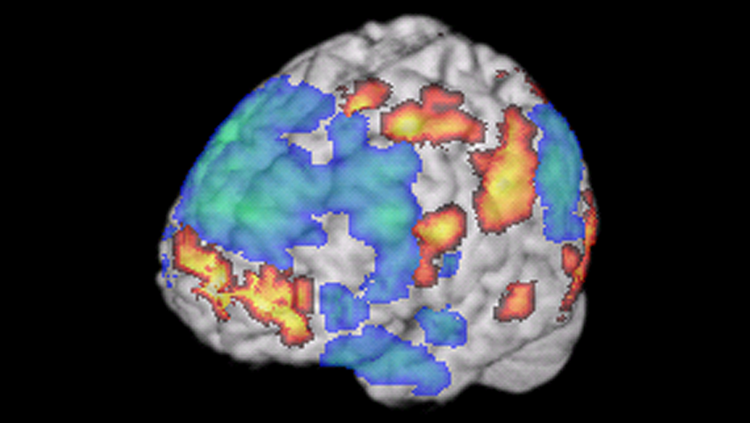
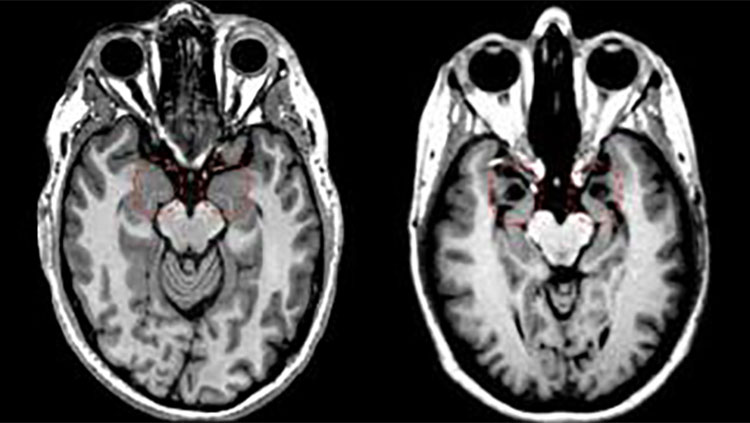
fMRI, or functional magnetic resonance imaging, uses a magnetic field to measure changes in blood flow in the brain. If a brain area is working hard to accomplish a certain behavior, it consumes more oxygenated blood, producing a dip in the fMRI signal. The simultaneous activation of multiple brain regions suggests the areas are talking to each other in a neural circuit. fMRI has revealed circuits underlying behaviors like reading or improvisation in jazz musicians, as shown here. fMRI is non-invasive — it doesn’t entail taking any drugs or undergoing any surgery.
Through an NIH BRAIN Initiative grant project, David Feinberg’s research team at the Helen Wills Neuroscience Institute at the University of California, Berkeley, is pushing the boundaries of fMRI by designing the NexGen 7T. This ultra-high-resolution imaging machine will be able to capture some of the smallest regions of the brain, providing non-invasive pinpoint views.
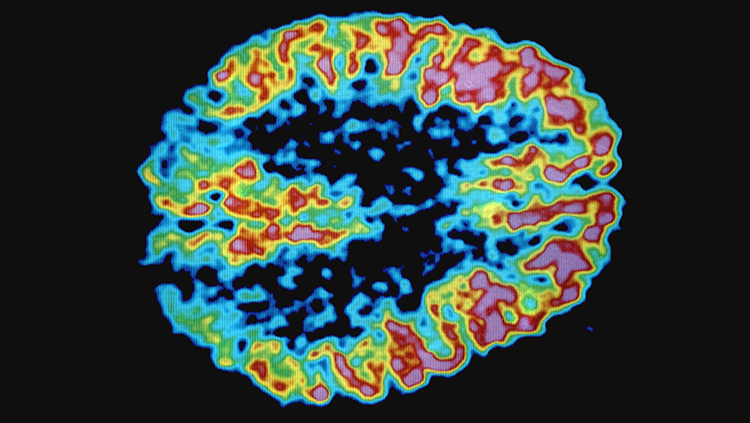
PET, or positron emission tomography, creates images of the brain by tracking radioactive molecules. Injected “radiotracers” — molecules like glucose or dopamine with radioactive atoms tacked onto them — enter the bloodstream and circulate throughout the body including the brain. A PET scanner can detect where these molecules end up in the brain.
For example, a glucose or oxygen radiotracer can identify which regions of the brain are the most metabolically active, revealing regions connected via circuits. A dopamine radiotracer can reveal which brain circuits communicate via that neurotransmitter.
Craig Levin’s research team at Stanford University was awarded a BRAIN Initiative grant to usher in the next generation of PET imaging technologies completely dedicated to the brain. The technology would heighten the sensitivity PET has in tracking the marked molecules and blend MRI-compatible interfaces.
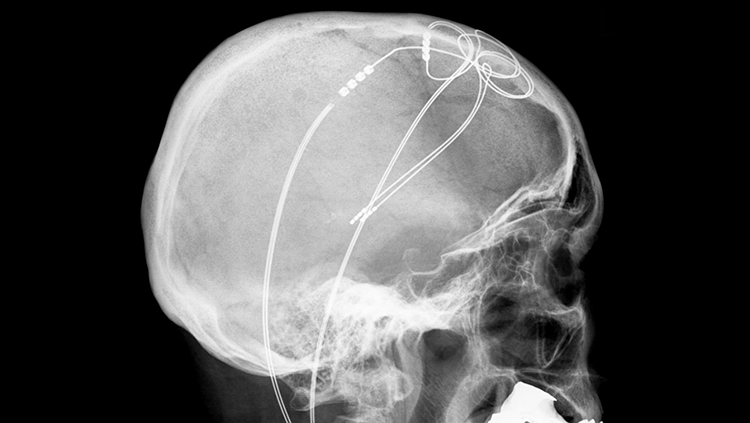
Deep brain stimulation (DBS) can reduce the symptoms of damaged neural circuits, like the tremors arising from the death of motor control cells in Parkinson’s disease. For DBS, a device must be implanted in the brain, so it is only used on patients who haven’t benefited from less invasive treatments like classic drug-based therapies. This device sends electricity into nearby brain tissue, which alters abnormal activity in neural circuits. Although scientists don’t fully understand how DBS works, it remains a reliable medical therapy that is particularly effective in treating Parkinson’s disease.
In a collaborative research effort, neuroscientists at the University of Maryland in Baltimore County are developing non-invasive technology to control neuron activity in targeted brain circuits to adjust the behavioral output and ultimately lead to therapies for brain diseases.

Transcranial direct (or alternate) current stimulation (tDCS/tACS) involves sending small electrical currents through the skull and into the brain. These currents produce subtle changes in neural circuits, but it is unclear how useful the technique really is for treating disorders in the brain. Some companies — and even the military — have explored using tDCS/tACS to improve normal cognition, but the results have been mixed.
However, Javier Medina and researchers at Baylor College of Medicine are investigating the unknowns of this technique to bridge the knowledge gap. Honing in on the cerebellum, a region of the brain known for its role in motor control, scientists are measuring the impact that tDCS has on neuron activity in different regions of the brain using mouse models.
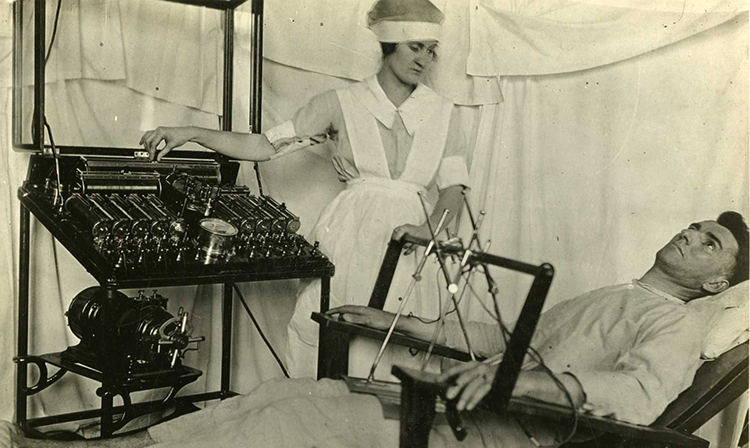
Electroconvulsive therapy (ECT) involves stimulating the brain with large amounts of electricity. It is one of the oldest techniques for treating problems in neural circuits. The large electrical currents used during ECT create short seizures, so patients who receive ECT must be monitored during treatment to ensure their safety. Despite its risks, ECT is sometimes useful for treating severe depression and other psychiatric illnesses. As with DBS, scientists do not know exactly how ECT works.
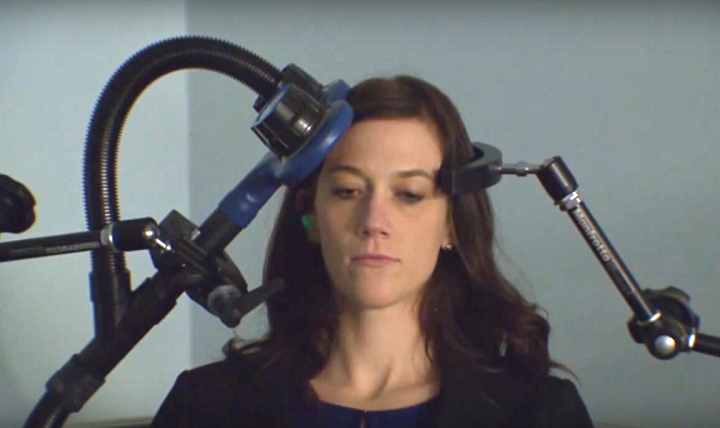
Transcranial magnetic stimulation (TMS) uses a strong magnetic field to create electrical currents in neural circuits. It merely requires placing a magnetic coil on top of the scalp. When used in a healthy person, TMS of certain parts of the brain can produce muscle movements. When TMS is used in people with damage to circuits controlling muscles, like stroke and multiple sclerosis patients, the expected muscle movements do not occur. TMS is also used to treat depression and neuropathic pain.
When we plan a movement, like picking up a pencil, our brains anticipate the sensory information we’ll receive — the weight, shape, and texture of the pencil, for example. Wilsaan Joiner’s team at the Sensorimotor Integration Laboratory at George Mason University is learning how we make these predictions and how we combine them with actual sensory feedback to adjust our movements on the fly. The effort, funded through a CAREER grant from NSF, will likely help scientists fine tune the timing and accuracy of brain-controlled neural prostheses.
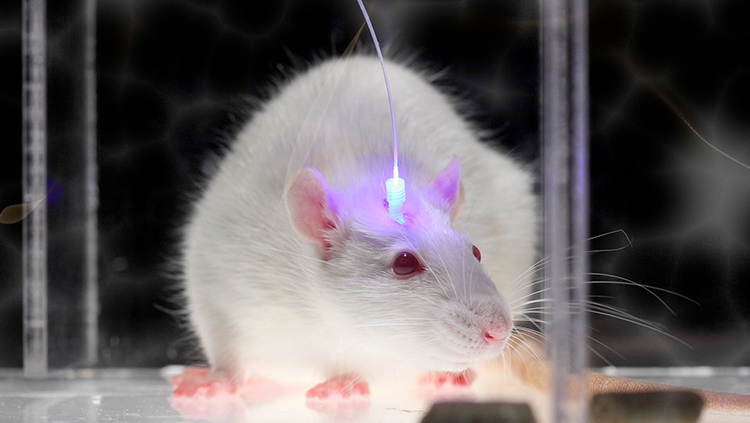
Optogenetics uses light-sensitive proteins and laser beams to alter the activity of neurons and circuits. When activated by a specific wavelength of light, the proteins turn on electrical signals in neurons. With optogenetics, scientists can switch neurons on and off and observe how it affects neural activity and behavior. The technology will allow scientists to understand the role each neuron plays in a circuit — a critical step in developing more targeted therapies for brain disorders.
With BRAIN EAGAR funding, Ian Wickersham and collaborators at the McGovern Institute for Brain Research at MIT are creating new optogenetics tools that target specific neurons in wild animal models — species that have not been genetically modified. These new tools, which will be made available to the scientific community, will help scientists study circuits with greater precision.
DREADDs (Designer Receptors Exclusively Activated by Designer Drugs) are proteins embedded in neurons that only respond to synthetic chemicals. When researchers inject the synthetic chemical, the neurons with DREADDs switch on. Unlike the proteins used for optogenetics, DREADDS do not need to be activated by lasers, so they can be used deep in the brain. Scientists use DREADDS to study how neurons in neural circuits process outside signals.
Currently, DREADD technology can only be used in mouse models. An interdisciplinary research team at the Icahn School of Medicine at Mount Sinai is investigating how to translate the next generation of DREADD technologies to humans.
CONTENT PROVIDED BY
BrainFacts/SfN
References
Rudorfer MV, Henry ME, Sackeim HA. Electroconvulsive therapy. In Tasman A, Kay J, Lieberman JA (eds) Psychiatry, Second Edition. Chichester: John Wiley & Sons, Ltd, 1865–1901 (2003).